Warnings About Levaquin and Avelox Strengthened by FDA
Topics: Health, FDA, Fluoroquinolones, FLQ Lawsuit, Fluoroquinolones Lawsuit, Fluoroquinolone, Avelox, Levaquin Lawsuit, FLQ, Fluoroquinolone Lawsuit, Levaquin, The Law Offices of Foster & Houston, Ryan Foster, Foster & Houston, Medical Update, Avelox Lawsuit
Federal Panel Addresses Concerns Over Dangerous Bleeding Related to Xarelto
In 2012, a federal advisory panel voted against expanding the use of the anticoagulant drug Xarelto. According to the New York Times, the panel found that dangerous bleeding associated with Xarelto outweighed evidence that the drug helps reduce the risk of blood clots.
Topics: Health, Health News, FDA, Health Study, Health Research, The Law Offices of Foster & Houston, FANDHLAW, Ryan Foster, Foster & Houston, Xarelto, Xarelto Lawsuit
Use of Pradaxa Restricted in Patients with Mechanical Heart Valves
Pradaxa should not be used to prevent stroke or blood clots in patients with medical heart valves, according to the U.S. Food and Drug Administration (FDA). In this safety communication the FDA states Pradaxa users with mechanical prosthetic heart valves are more likely to experience stroke, heart attack, and blood clots than Warfarin users.
Topics: Health, Health News, Pradaxa, FDA, Health Study, Health Research, The Law Offices of Foster & Houston, FANDHLAW, Pradaxa Lawsuit, Ryan Foster, Foster & Houston, Mechanical Heart Valves
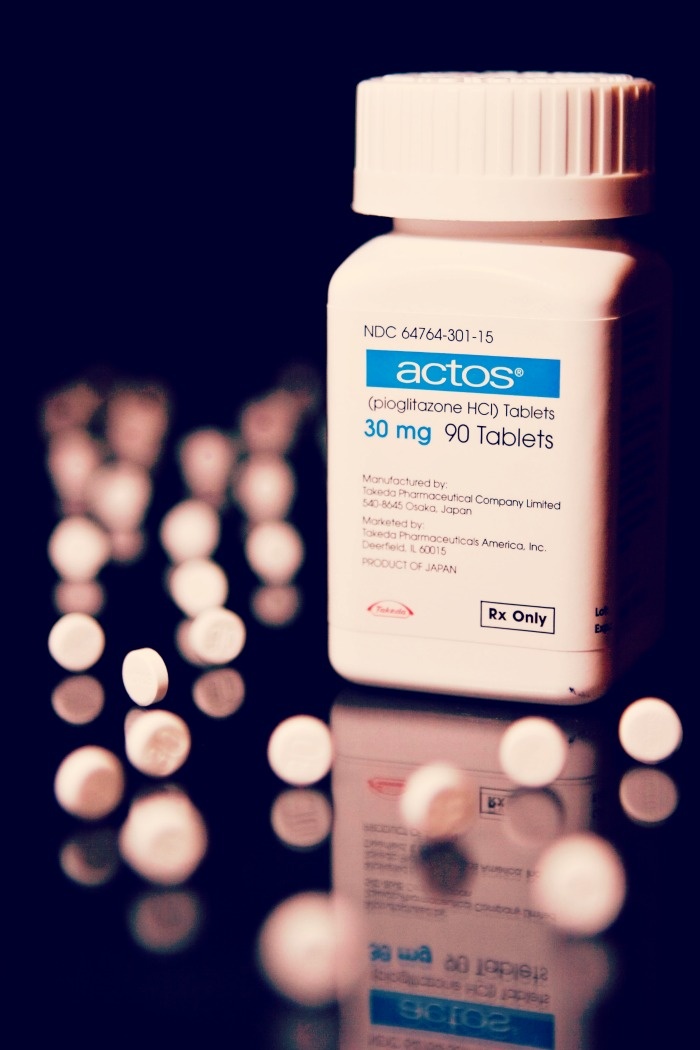
Takeda Pharmaceutical Co., the manufacturer of Actos, proposed $2.2 billion to settle thousands of lawsuits, according to Bloomberg. As of April 2015 more than 8,000 lawsuits had been filed against the diabetes medicine claiming hidden cancer risks.
Topics: Health, Actos, FDA, Actos Lawsuit, Diabetes, Diabetes Drug, The Law Offices of Foster & Houston, FANDHLAW, Ryan Foster, Foster & Houston, Diabetes Drug Lawsuit
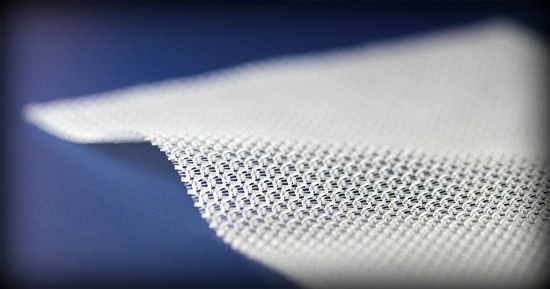
Complications associated with transvaginal placement of pelvic mesh can have serious consequences, according to the FDA. Typically used to repair damaged or weakened tissue, surgical mesh is typically used for pelvic organ prolapse (POP) or stress urinary incontinence surgery.
Topics: Health, FDA, Surgical Mesh, Transvaginal Mesh, Transvaginal Mesh Lawsuit, The Law Offices of Foster & Houston, Mesh Lawsuit, Surgical Mesh Lawsuit, FANDHLAW, Ryan Foster, Foster & Houston
After a recent safety review, the FDA called for restricted use of fluoroquinolones, such as Levaquin and Avelox, in certain patients. These new recommendations come after an expert advisory panel met to review evidence on the safety of using fluoroquinolones to help treat common infections.
Topics: Health, FDA, Fluoroquinolones, FLQ Lawsuit, Fluoroquinolones Lawsuit, Fluoroquinolone, Avelox, Levaquin Lawsuit, FLQ, Fluoroquinolone Lawsuit, Levaquin, The Law Offices of Foster & Houston, Ryan Foster, Foster & Houston, Medical Update, Avelox Lawsuit
FDA Claims Zofran may be Associated with Abnormal Heart Rhythms
In 2011, the U.S. Food and Drug Administration (FDA) issued a safety communication stating the anti-nausea drug Zofran may increase the risk of potentially fatal abnormal heart rhythms. Patients at particular risk of changes in the electrical activity of the heart include those with underlying heart conditions.
Topics: Health, Medical, Medical News, FDA, The Law Offices of Foster & Houston, FANDHLAW, Zofran Lawsuit, Zofran, Ryan Foster, Foster & Houston